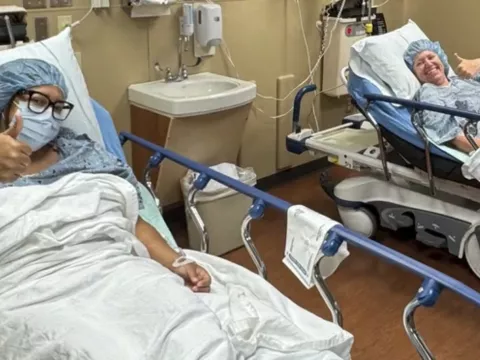
- Kristi Powers
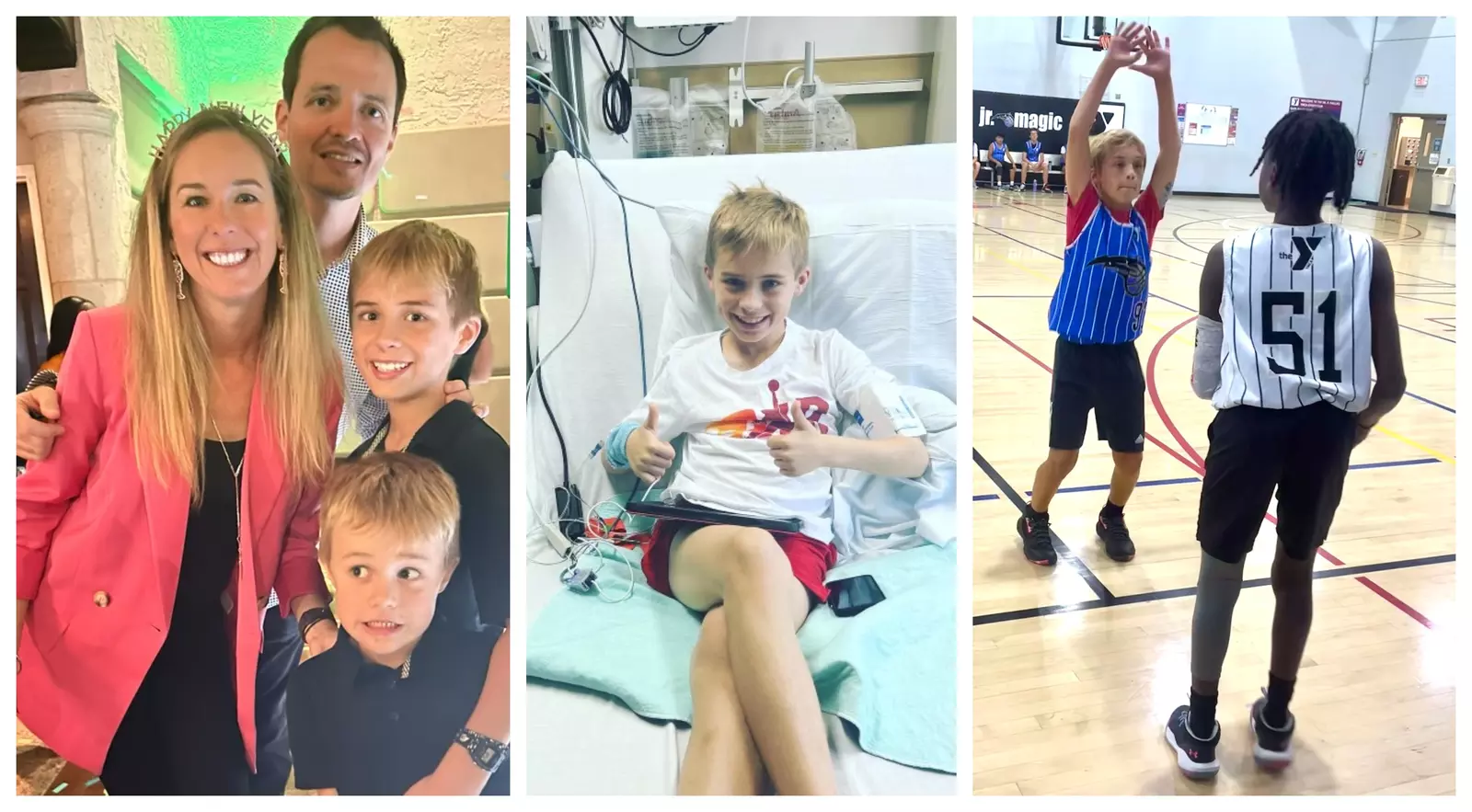
Anderson Ata is a typical 10-year-old who likes playing basketball and hanging out with his friends, but on New Year’s Eve he got really sick.

“I took a couple of covers from my cabinet and put them on me, waited about an hour and I was still shaking and I didn’t know what was happening,” Anderson Ata explained to FOX 35 News Anchor Amy Kaufeldt.
Doctors diagnosed Anderson with early stages of Type 1 diabetes. The family was referred to Dr. Konda Reddy to administer the life-changing monoclonal antibody treatment. Anderson became the seventh patient in the world, and the first in Orlando, to receive the new FDA-approved drug called Tzield.
To determine eligibility for the treatment, a simple blood test is performed. TZIELD, is the first drug that can delay the progression of Type 1 diabetes by up to three years in adults and up to eight years in children.
AdventHealth was a part of a large global research study which led to TZIELD gaining FDA-approval. When TZIELD became available on the market at the beginning of the year, AdventHealth was one of a handful of hospital systems administering the groundbreaking treatment.
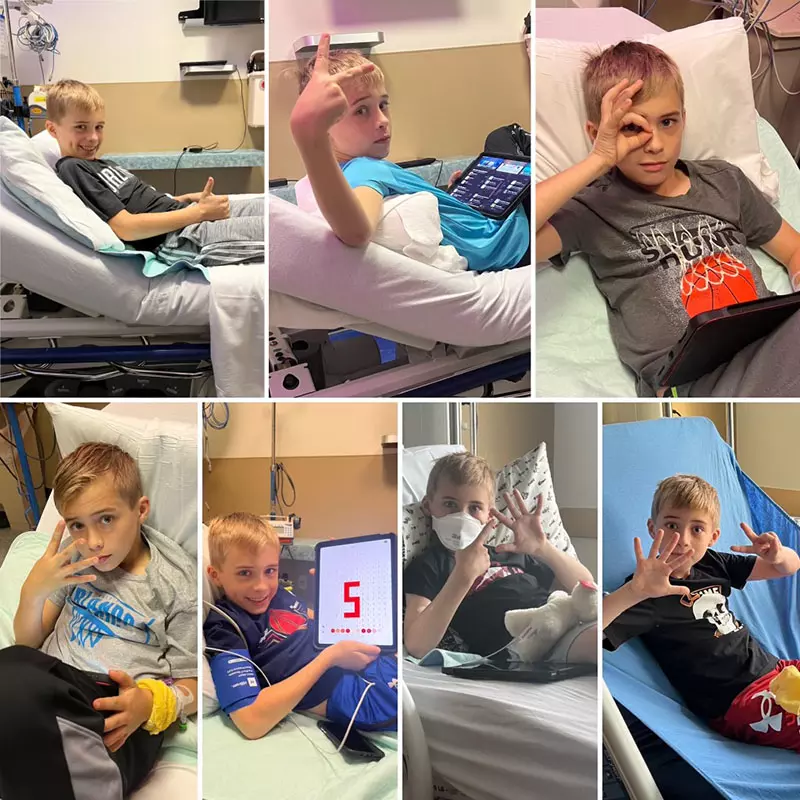
Learn more about how the treatment is given and how Anderson is doing post treatment, here.
Choose the health content that’s right for you, and get it delivered right in your inbox.
Recent News
The Inspiring Wholeness podcast explains how to start an exercise routine, stay motivated and build endurance safely, to find your inner Ironman.
As the world rang in 2025, AdventHealth for Women welcomed the very first babies of the new year.
Dr. Joseph Lopez, chief of pediatric head and neck surgery at AdventHealth for Children, was honored with the prestigious Professional of the Year Award at the 27th Annual Don Quijote Awards.
Giving back to his hometown, Dr. Ryan Day brings advanced robotic surgery to local patients, offering life-saving care close to home.
The holiday season can increase heart attack risks due to overindulgence, stress, and ignored symptoms, but Dr. Hector Lozano advises moderation, staying active, managing stress, and sticking to...
Transplant is AdventHealth Transplant Institute’s 5000th kidney transplant
Deputies from local fire and police departments dressed as elves as part of an eight-year long tradition bringing festive cheer to kids and families staying at the hospital this holiday season.
Deputies from local fire and police departments dressed as elves and dropped in to visit patients as part of an eight-year long tradition bringing festive cheer to kids and families staying at the...
AdventHealth is now using a fluorescent dye that lights up cancer cells during surgery, which is providing faster, more accurate treatment for patients.
The Ahn family’s life was turned upside down when their 5-year-old daughter suddenly started having 30 seizures a day. She had FIRES Syndrome, a one-in-a-million and potentially fatal diagnosis.
Investments will fund affordable housing in Bithlo and Seminole County, as well as job training programs at Special Hearts Farm and projects with The Salvation Army of Orlando and Cristo Rey Orlando...
On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.