- AdventHealth
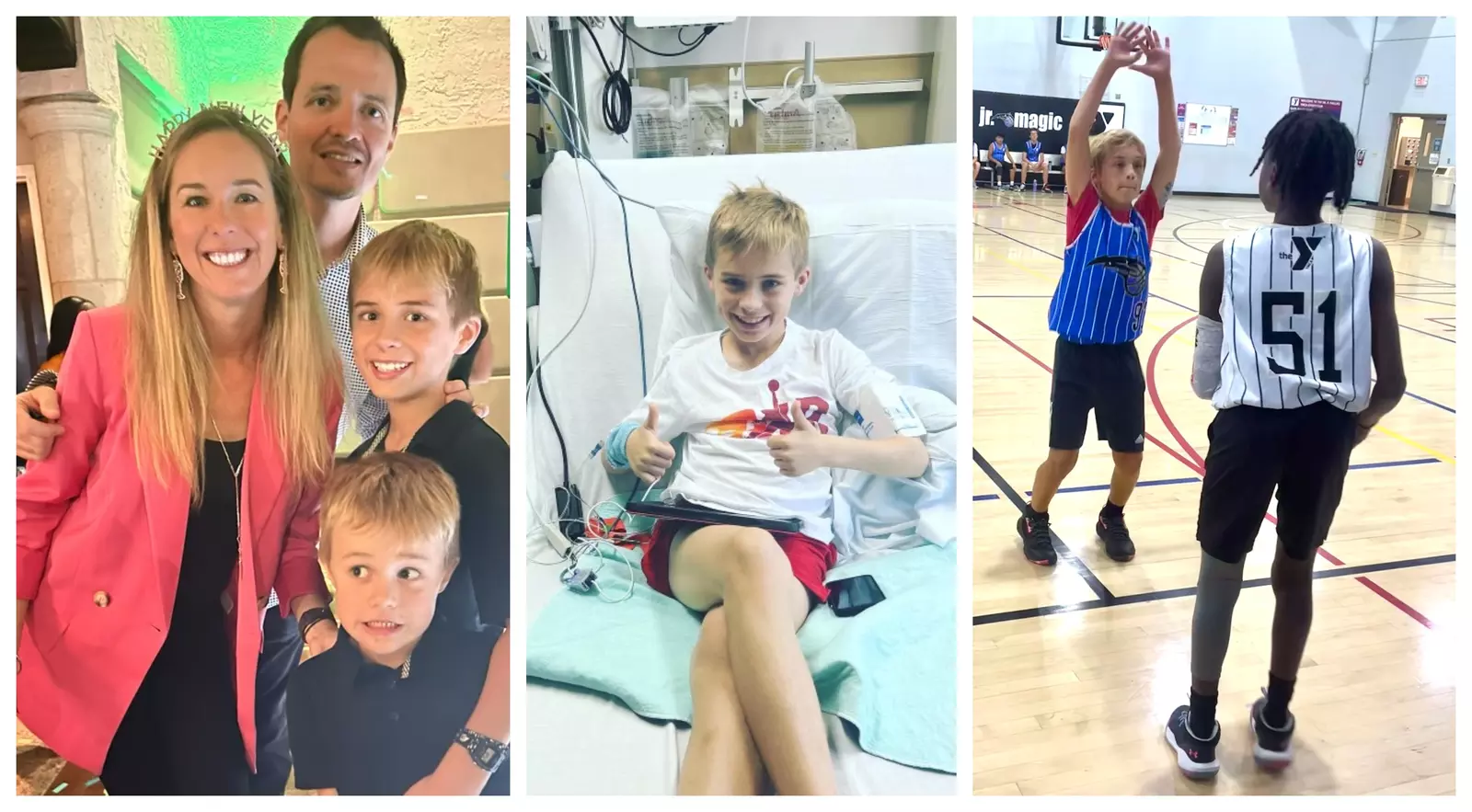
It has been a little over a year since Anderson Ata, a typical 11-year-old who likes playing basketball and hanging out with his friends, got really sick.
During the night of New Year’s Eve 2022, Anderson said, “I took a couple of covers from my cabinet and put them on me, waited an hour and I was still shaking, and I didn’t know what was happening.” Anderson shared his story with FOX 35 news.
After an ER visit last January, Anderson learned he had the early stages of Type 1 diabetes. The family made an appointment with Konda Reddy, MD, medical director of pediatric endocrinology and diabetes at AdventHealth for Children, to explore possible treatment with a brand-new FDA-approved drug called TZIELD.
TZIELD is the first drug that has been shown to delay the progression of Type 1 diabetes by up to three years in adults and up to eight years in children. Anderson became the seventh patient in the world, and the first in Florida to receive the life-changing treatment.
Just recently, Anderson and his parents, Joe and Jill Ata, shared with WMFE the good news that, a year later, Anderson shows no symptoms.
AdventHealth was part of the large global research study that led to the FDA’s approval of TZIELD. Because of its participation, AdventHealth’s Central Florida Division, along with a handful of other hospital systems, was among the first in the world to administer TZIELD, which is a 14-day intravenous treatment, when it became available at the beginning of 2023.
Recent News
On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.
AdventHealth President and CEO Terry Shaw has been named one of Modern Healthcare’s 100 Most Influential People in Healthcare for 2024, marking continued recognition of Shaw as a transformative leader...
AdventHealth President/CEO Terry Shaw's retirement is planned for July 2025.
On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.
AdventHealth has named Elise MacCarroll-Wright as president and CEO of UChicago Medicine AdventHealth Hinsdale in its Great Lakes Region, effective Jan. 6, 2025.
AdventHealth has named Dave Tkachuck president/CEO for UChicago Medicine AdventHealth La Grange, effective Jan. 6.
A UChicago Medicine AdventHealth neurologist addresses the growing gap between the number of men and women diagnosed with MS.
AdventHealth has named Ryan Quattlebaum president/CEO for AdventHealth Wesley Chapel, effective Dec. 29.
Health care and government leaders celebrate the much-anticipated expansion of AdventHealth University.
Wangsness has more than 30 years of experience in health care.









