- AdventHealth
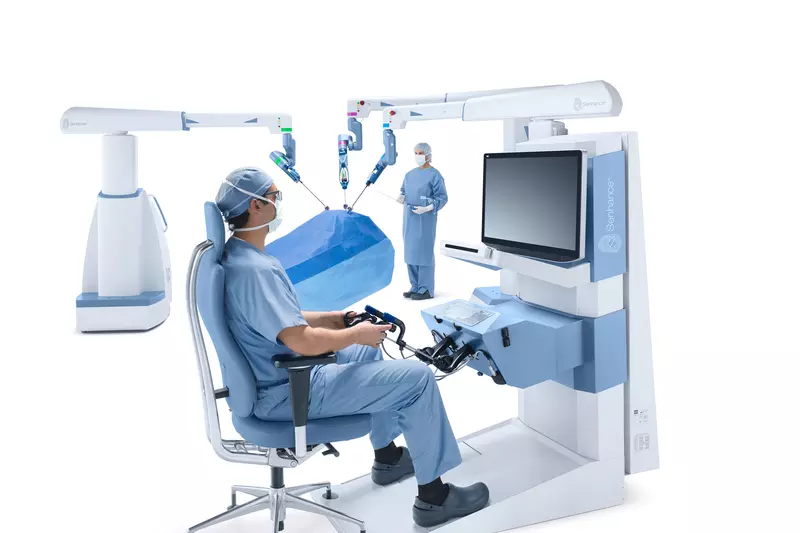
AdventHealth is proud to partner with TransEnterix on the introduction of a new surgical robotic system, available to patients in the United States for the first time.
The Senhance Surgical System, which is currently in use in Europe, received FDA approval in October. Its the first new robotic surgical system to receive this approval since 2000. AdventHealth will be the first U.S. health-care system to utilize the Senhance, both for training purposes and for clinical use.
The controls for the Senhance mimic traditional laparoscopic surgery, allowing for an accelerated adaptation for surgeons currently performing surgeries with these tools. The surgeon sits in an ergonomically comfortable position and can see inside the body with 3D visualization. Optical sensors allow surgeons to move the camera and select commands simply by moving their eyes. The Senhance also is the first robotic system that offers haptic feedback, which re-creates the sense of force feedback for surgeons so that they can feel forces encountered via the systems robotic arms.
AdventHealth is always seeking out innovative technology to advance patient care, said Dr. Steve Eubanks, executive medical director of the AdventHealth Institute for Surgical Advancement at AdventHealth. We are very pleased to partner with the TransEnterix team to help train surgeons on the Senhance and begin using it in the coming months to treat patients in our community.
Several AdventHealth surgeons have already completed their training on the Senhance and are looking forward to beginning clinical procedures soon. The system will benefit patients across a range of procedures and specialties, including gynecology and colorectal surgery.
The FDA approval of Senhance in the United States and the early adoption of the system at AdventHealth are milestones in the progress of robotics, said Todd M. Pope, president and CEO of TransEnterix, Senhance's manufacturer. Senhance will strengthen the senses, precision and comfort of the surgeon, minimize the invasiveness of surgery for the patient, and maximize value for the hospital.
The Senhance's instruments are just 5 millimeters in diameter, and the system senses and minimizes forces at the patients’ small skin incisions. As a result, current minimally invasive surgery may become even more so, which means quick and easy recovery for patients, with little to no scarring.
And the Senhance allows for the reuse of surgical instruments, helping to lower the overall cost of procedures, and in turn, health care overall.
For media inquiries only, call AdventHealth Corporate Communications at Call407-303-5950.
About AdventHealth
AdventHealth operates 45 hospitals in 9 states, making it one of the largest not-for-profit healthcare systems in the United States, caring for more than 5 million patients annually. AdventHealth continues to be at the forefront of health-care innovation. In addition to the AdventHealth Institute for Surgical Advancement, it’s also home to the AdventHealth Nicholson Center, a facility specializing in training surgeons from around the globe on the latest surgical technology and techniques, and the Global Robotics Institute, a world leader in robotic-assisted treatment of prostate cancer.
About TransEnterix
TransEnterix is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options. The company is focused on the commercialization of the Senhance Surgical Robotic System, a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology such as haptic feedback and eye sensing camera control. The company is also developing the SurgiBot System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance Surgical System has received FDA 510(k) clearance and has been granted a CE Mark. For more information, visit the TransEnterix website at www.transenterix.com.
Recent News
-
AdventHealth’s 18th annual Sporting Clay Shoot raised over $45,000 to fund scholarships for future AdventHealth University students.
-
AdventHealth Cancer Institute’s rectal cancer program has received a three-year certification by the National Accreditation Program for Rectal Cancer (NAPRC). This accreditation, currently held by...
-
Orlando Colorectal Congress (OCC) features three days of didactic sessions with international guest lecturers as well as DLive Med case demonstrations, and cadaveric labs for demonstrating advanced...
